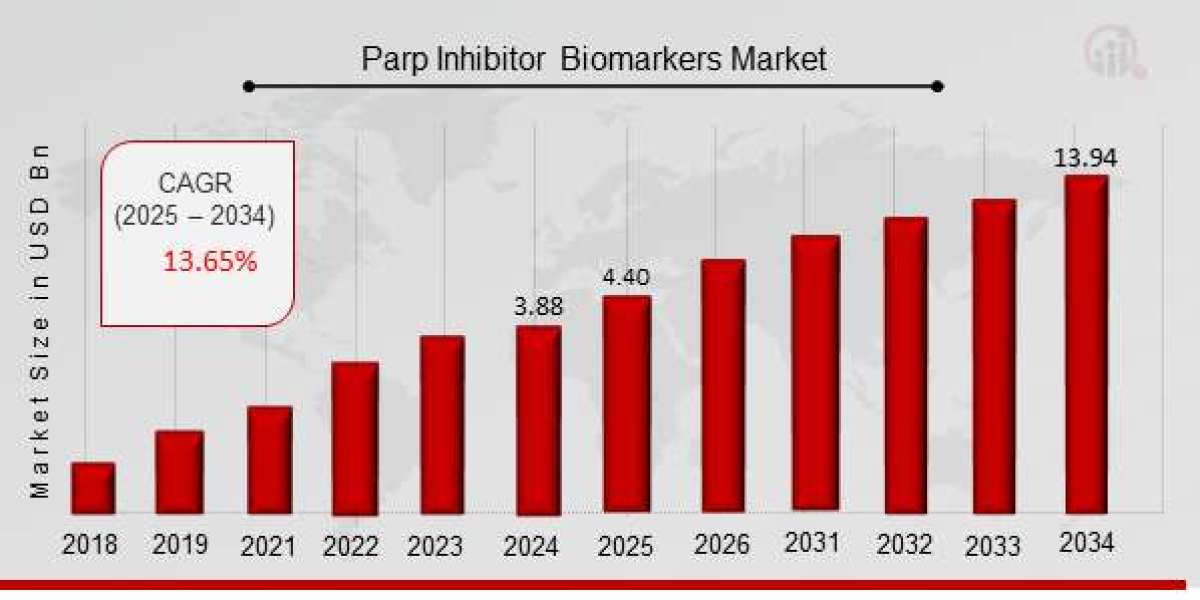
The Future of PARP Inhibitor Biomarkers in Cancer Treatment by 2025
In recent years, the world of oncology has seen tremendous breakthroughs, thanks in part to the development of PARP inhibitors (Poly(ADP-ribose) polymerase inhibitors), a class of drugs that are transforming cancer treatment. PARP inhibitors are designed to target and block the PARP enzyme, which plays a critical role in DNA repair mechanisms. By inhibiting this enzyme, these drugs selectively kill cancer cells, especially those with DNA repair deficiencies, making them an effective treatment option for several cancers, including ovarian, breast, prostate, and pancreatic cancers.
However, while PARP inhibitors have shown significant promise in clinical trials and early-stage treatments, their success heavily depends on identifying patients who will benefit the most from this therapy. This is where PARP inhibitor biomarkers come into play. Biomarkers are biological molecules that indicate the presence or progression of a disease and can also predict how a patient will respond to a particular treatment. By 2025, the use of PARP inhibitor biomarkers is expected to revolutionize personalized cancer therapy, improving both treatment efficacy and patient outcomes.
What Are PARP Inhibitor Biomarkers?
PARP inhibitor biomarkers are genetic or molecular markers that help identify which cancer patients will respond favorably to treatment with PARP inhibitors. These biomarkers are typically associated with mutations or alterations in DNA repair pathways, most notably in genes such as BRCA1 and BRCA2, which are commonly linked to breast and ovarian cancers.
When a tumor carries specific mutations in DNA repair genes, it becomes more dependent on PARP to repair its damaged DNA. By inhibiting PARP, these tumors lose their ability to repair DNA, leading to cell death. As such, identifying patients who have these mutations is key to determining whether PARP inhibitors will be effective for them.
In addition to BRCA mutations, other biomarkers, such as HRD (Homologous Recombination Deficiency), ATM mutations, and PALB2 mutations, are also being explored to predict the efficacy of PARP inhibitors. These biomarkers are paving the way for personalized medicine, allowing oncologists to tailor treatments based on the genetic makeup of a patient’s tumor.
The Role of PARP Inhibitor Biomarkers in Personalized Medicine
One of the most significant advancements in oncology is the move toward personalized medicine, which tailors treatments to the specific genetic profile of an individual’s cancer. PARP inhibitors have emerged as a prime example of how biomarker-driven therapies are transforming cancer treatment.
Currently, many cancers are treated using broad-spectrum approaches that may not work equally well for all patients. However, with the use of PARP inhibitor biomarkers, doctors can identify which patients are more likely to respond positively to these drugs. For example, patients with BRCA1/2 mutations or other DNA repair deficiencies are more likely to benefit from PARP inhibitors compared to those without these mutations.
In the future, the use of PARP inhibitor biomarkers will be central to ensuring precision medicine. By 2025, we can expect a more widespread use of genetic testing to identify patients who will benefit from PARP inhibitors. This will not only maximize the therapeutic potential of the drug but also minimize unnecessary treatments and side effects for those who are unlikely to respond.
Clinical Trials and Advancements in Biomarker Discovery
The search for new PARP inhibitor biomarkers is ongoing. Researchers are constantly exploring how different genetic mutations and molecular markers could affect a patient's response to treatment. By 2025, advancements in genomic sequencing and more comprehensive clinical trials will likely lead to the discovery of additional biomarkers that can predict PARP inhibitor efficacy across a broader range of cancers.
For example, trials are underway to identify new biomarkers in cancers such as pancreatic and prostate cancer, where the use of PARP inhibitors has shown promise but is not yet widely established. Understanding which patients in these cancer types will benefit most from PARP inhibitors will open up new treatment options for patients with previously limited therapeutic choices.
Additionally, liquid biopsy technology—a less invasive method of testing for biomarkers—will become more advanced and widely used in clinical settings. Liquid biopsies allow for the detection of genetic mutations and other biomarkers from blood samples, making it easier to identify potential candidates for PARP inhibitor therapy without the need for tissue biopsies. This could greatly enhance the accessibility and speed of biomarker testing, leading to quicker treatment decisions and better outcomes for cancer patients.
The Impact of Biomarker-Driven PARP Inhibitor Treatment on Cancer Outcomes
The ultimate goal of integrating PARP inhibitor biomarkers into cancer treatment is to improve patient outcomes. By accurately identifying which patients are likely to benefit from these drugs, doctors can provide more effective treatments, reduce unnecessary side effects, and potentially improve survival rates.
Moreover, the biomarker-guided use of PARP inhibitors will likely play a role in extending the use of these drugs beyond their current indications. For instance, ongoing research into PARP inhibitors is exploring their potential in combination with other treatments, such as immunotherapy and chemotherapy. Identifying the right biomarkers to guide combination therapies could lead to more powerful, synergistic treatment regimens.
As more patients benefit from biomarker-driven therapies, it could lead to broader acceptance and adoption of PARP inhibitors in clinical oncology. By 2025, these therapies may become a cornerstone of cancer treatment, especially for patients with specific genetic mutations or deficiencies in DNA repair.
Conclusion: The Promising Future of PARP Inhibitor Biomarkers
The future of PARP inhibitors in cancer treatment is bright, and the role of biomarkers in this field will only continue to grow. By 2025, the combination of advances in genomic research, personalized medicine, and clinical trials will result in the identification of more precise biomarkers, allowing for even more effective and targeted treatments. With the help of these biomarkers, PARP inhibitors will help oncologists deliver personalized therapies that maximize patient outcomes while minimizing side effects.
As the market for PARP inhibitors continues to expand, and as we gain a deeper understanding of how different biomarkers impact treatment efficacy, the potential to significantly improve cancer survival rates is within reach. The continued development of biomarker-based therapies represents a promising step toward more effective, individualized cancer treatments in the years to come.
This blog post explores how PARP inhibitor biomarkers are revolutionizing cancer treatment, providing insights into the role of these biomarkers in personalized medicine and their future impact on cancer therapies.