Building the Future of Medicine: The Nucleic Acid Therapeutics CDMO Market
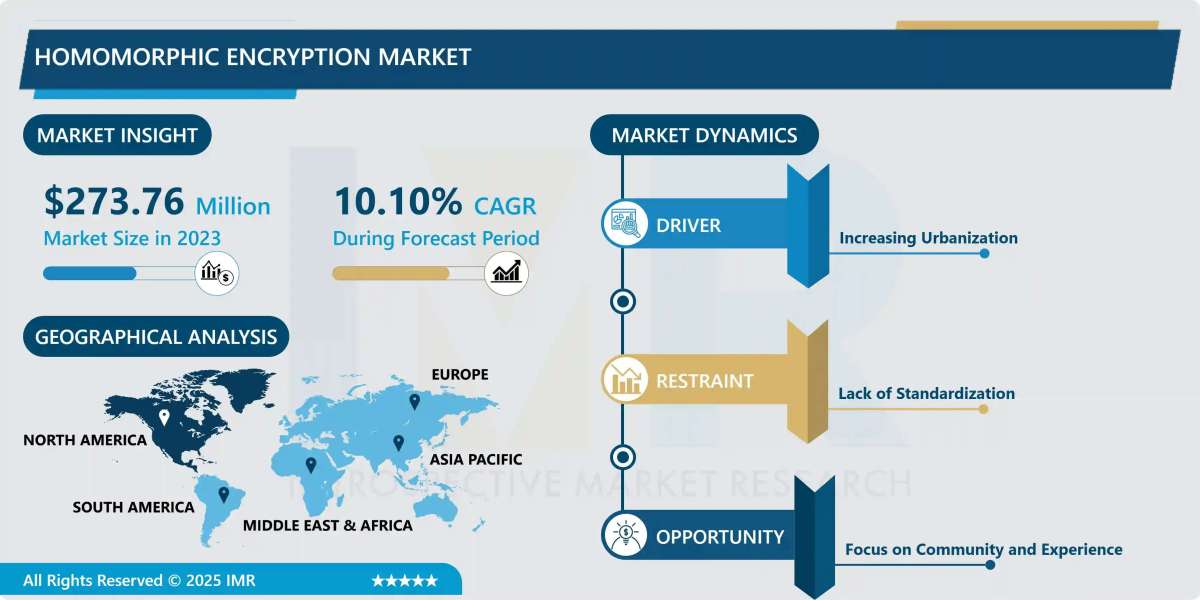
The Nucleic Acid Therapeutics Contract Development and Manufacturing Organization (CDMO) Market is a critical and rapidly expanding sector that provides specialized services for the development and manufacturing of nucleic acid-based drugs. These groundbreaking therapies, including RNA interference (RNAi), antisense oligonucleotides (ASOs), messenger RNA (mRNA) therapies, and CRISPR-based gene editing, are revolutionizing medicine by targeting diseases at the genetic level. CDMOs in this space offer a comprehensive range of services, from process development and analytical testing to large-scale GMP (Good Manufacturing Practice) manufacturing, enabling pharmaceutical and biotech companies to bring these complex, innovative drugs to market. The global nucleic acid therapeutics CDMO market size was valued at approximately USD 2.54 billion in 2024 and is projected to reach USD 8.68 billion by 2034, reflecting a robust CAGR of 13.2% during the forecast period.
Robust Pipeline and Specialized Expertise as Key Driver
Several powerful factors are propelling the growth of the Nucleic Acid Therapeutics CDMO Market. The most significant driver is the explosive growth in the pipeline of nucleic acid-based drugs. These novel therapies offer unprecedented potential for treating a wide range of conditions, including genetic disorders, infectious diseases (as demonstrated by mRNA vaccines), and various cancers. As more nucleic acid drugs advance through clinical trials and gain regulatory approval, the demand for specialized manufacturing services surges. Developing and manufacturing these complex molecules requires highly specialized knowledge, significant capital investment in advanced facilities, and a deep understanding of unique purification and quality control challenges. Many biopharma companies opt to outsource these activities to CDMOs to leverage their expertise, manage high upfront capital expenditures, accelerate time-to-market, and scale production efficiently. Furthermore, increasing R&D investments in genomics research and a growing preference for personalized medicine are further fueling the demand for custom oligonucleotide synthesis and specialized manufacturing.
Key Services and Technological Advancements
The Nucleic Acid Therapeutics CDMO Market is highly dynamic, segmented by various services and technologies:
Services:
Manufacturing Services: This segment currently accounts for the largest market share, driven by the increasing need for highly specialized knowledge and expertise in the production of nucleic acid therapies, from small-scale clinical batches to large-scale commercial production.
Process Development: Optimizing synthesis, purification, and formulation.
Analytical Testing: Ensuring purity, potency, and stability.
Custom Nucleic Acid Synthesis: For research and early-stage development, representing a significant portion of the market.
Technology:
Column-Based Method: A traditional synthesis mode widely used, particularly for custom oligonucleotide production.
Microarray-Based Method: Emerging for high-throughput applications.
Solid-Phase Oligonucleotide Synthesis (SPOS): Dominates the global market, allowing for efficient purification and automation.
Product Types: Standard Nucleic Acid, Micro-Scale Nucleic Acid, Large-Scale Nucleic Acid, Custom Nucleic Acid, Modified Nucleic Acid, Primers, Probes, and Other Nucleic Acids.
End-Users: Pharmaceutical companies (dominant), biotechnology companies, academic research institutes, and diagnostic laboratories.
Technological advancements are continuously shaping this market. Innovations in high-throughput oligonucleotide synthesis, scalable in vitro transcription platforms, and advanced purification techniques (e.g., tangential flow filtration) are critical. The integration of Process Analytical Technologies (PAT) and real-time quality monitoring significantly enhances manufacturing reliability and reduces batch failures. Furthermore, the adoption of automation and robotics is streamlining complex processes, while AI and machine learning algorithms are being explored to predict critical quality attributes and accelerate process validation.
Challenges and Regional Dominance
Despite the robust growth, the Nucleic Acid Therapeutics CDMO Market faces certain challenges. Scaling up production to meet commercial demand while maintaining stringent quality and cost-effectiveness remains a significant hurdle. The complexity of nucleic acid molecules and the need for specialized equipment and expertise limit the number of capable CDMOs. Furthermore, supply chain challenges for critical raw materials and reagents can impact production timelines and costs.
Geographically, North America currently dominates the market with the largest revenue share in 2024, driven by a strong biopharmaceutical industry, significant R&D investments, and a robust regulatory framework. The Asia-Pacific region is anticipated to exhibit high growth due to increasing investments in biotech, growing outsourcing trends, and a rising prevalence of chronic and genetic diseases.
The Future Outlook for Nucleic Acid Therapeutics CDMOs
The future of the Nucleic Acid Therapeutics CDMO Market is characterized by sustained high growth and increasing strategic importance. As nucleic acid therapies continue to move from niche treatments to mainstream medicines, the demand for specialized CDMO services will intensify. We can expect further consolidation in the market as larger CDMOs acquire smaller, specialized players to expand their capabilities and capacity. Investment in advanced manufacturing technologies, including continuous manufacturing solutions, will also be a key trend. Ultimately, CDMOs will play an increasingly vital role as strategic partners, enabling the efficient, high-quality, and cost-effective production of these transformative medicines, bringing hope to patients with previously untreatable conditions.
Market Research Future®
99 Hudson Street,5Th Floor
New York, New York 10013
United States of America
Phone:
+1 628 258 0071(US)
+44 2035 002 764(UK)
Email: sales@marketresearchfuture.com
Website: https://www.marketresearchfuture.com